COMPANY
COMPANYQuality Assurance
At Biotoxtech, our Quality Assurance team ensures quality and reliability.
- GLP Compliance
- Continuous quality improvement
- Risk-based Quality Assurance

Inspections and Audits
· Study-based Inspection
· Process-based Inspection
· Facility-based Inspection
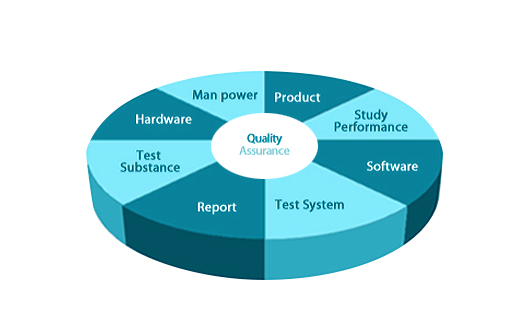
Role of Quality Assurance
Biotoxtech establishes SOP with rational understanding of GLP and guarantee accuracy of data through the observing SOP.
QA & main roles
The goal of QA is a building trusted report for manufacture, per-mission and application of drugs, food, cosmetics, cell thera-peutics, chemicals and agrochemicals. The person who is a member of QAU and nominated by operating director inspects facilities, equipments, investigators, proto-cols and record.